Today, let's take a glance at Qiming Venture Partners' portfolio company CanSinoBIO (SEHK:6185, SHSE:688185)'s inhaled COVID-19 vaccine Convidecia Air™.
As age increases, the elderly have weaker autoimmunity and tend to have underlying diseases, making them a high-risk group vulnerable to COVID-19.
According to the analysis of morbidity, severe illness and death of COVID-19 monitored in countries worldwide, people over 60 are at high risk of severe illness and death. Because they require hospitalization with a high proportion of emergency treatments, as well as longer hospital stays, the elderly take up most healthcare resources during the pandemic.
According to the mortality data of people infected with the Omicron variant in Hong Kong SAR, the mortality rate of those who were not vaccinated was much higher than those who were vaccinated, with a discrepancy of more than 10 times.
Therefore, in order to reduce the rate of severe illness and mortality in the elderly and to defend the health system, the World Health Organization (WHO) recommends that the elderly be a priority population for vaccination.
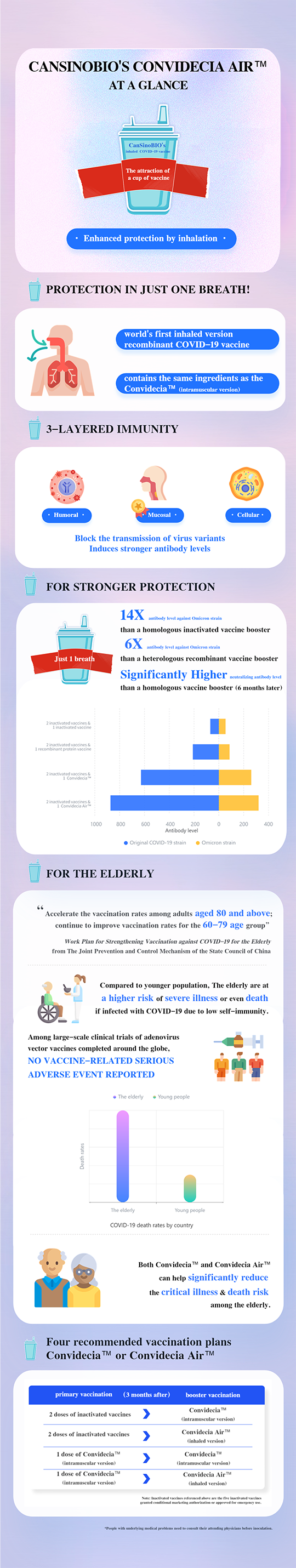
On November 29, China's State Council Integrated Group for Joint Prevention and Control Mechanism for Response to COVID-19 issued the "Work Plan for Strengthening the COVID-19 Vaccination of Elderly People" (hereinafter referred to as the "Work Plan"), which calls for further accelerating the vaccination of elderly people to give full force to the protective effect of COVID-19 vaccines, reducing the risk of severe, critical illness and death among the elderly after infection with COVID-19 viruses.
The work plan recommended six vaccination combinations, four of which included CanSinoBIO's intramuscular and inhaled vaccines. Among them, the world's first inhaled COVID-19 vaccine - Recombinant COVID-19 Vaccine (Adenovirus Type 5 Vector) for Inhalation (trade name: Convidecia Air™) independently developed by CanSinoBIO, is currently available for booster immunization of the elderly.
The inhaled vaccine does not require a needle and is administered through "oral inhalation of the respiratory tract". This vaccine stimulates mucosal immunity in the respiratory tract, compared to the intramuscular vaccine. Studies have shown that respiratory mucosal immunity is a more effective method of preventing infection and interrupting transmission.
Another clinical study showed that booster immunization with inhaled vaccine on top of 2 doses of inactivated vaccine resulted in 14 times more neutralizing antibody levels against the Omicron variant than homologous boosters of inactivated vaccines; 6 times more than sequential boosters of recombinant protein vaccine. Six months after the booster, the neutralizing antibody level remained much higher than that of the homologous booster.
It is worth mentioning that people can still do nucleic acid testing after vaccination with the inhaled vaccine. Because it does not contain COVID-19 component, the inhaled vaccine will neither cause false positive test results of the surrounding environment during vaccine nebulization nor affect the recipient's test results after vaccination.
In September 2022, under the National Health Commission's recommendation, the National Medical Products Administration of China granted CanSinoBIO approval for its inhaled vaccine to be used as a booster dose. Later on, Shanghai, Tianjin, Beijing and several cities in Jiangsu and Zhejiang province rolled out the booster vaccination plan for their residents.
Elderly people who meet the vaccination requirements are encouraged to actively finish their all-course inoculation and take booster shots as soon as possible to protect themselves and their families.